Explain What the Different Colors in a Line Spectrum Represent
The lights travel on different line of spectrum that produce different color. Because an absorbed wavelength of light removes a color from the original continuous spectrum the resulting absorption spectrum is.
Why are the spectra for each element unique.
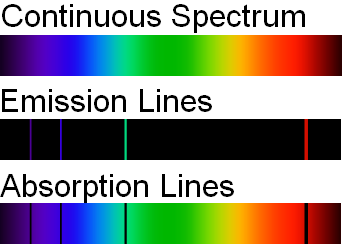
. They represent the wavelengths of light that is absorbed by the spectrum They represent the energy that the atom gives off when their electrons are in place. Hydrogen absorption and emission lines in the visible spectrum. The line spectra of several elements are shown in Figure 143.
Because of this a line emission spectrum is like the fingerprint of the element which we can use to identify the element. Line emission spectrum. Thus we can make different colors from elements that produce various colors.
Light outside of this range may be visible to other organisms but cannot be perceived by the human eye. A bright line emission spectrum shows only the colors that are associated with and emitted from a substance. Orange has different tones and shades each with different meanings and effects.
When atoms are excited they emit light of certain wavelengths which correspond to different colors. A spectral line is like a fingerprint that can be used to identify the atoms elements or molecules present in a star galaxy or cloud of interstellar gasIf we separate the incoming light from a celestial source using a prism we will often see a spectrum of colours crossed with discrete lines. The emitted light analyzed by a spectrometer or even a simple prism appears as a multitude of narrow bands of color.
Asphalt roads for example are dark blue or black. You dont see all the wavelengths. The marks that you make on the three color lines will represent the different wavelengths of the different colors of light.
The electromagnetic spectrum is a range of frequencies of different energy waves such as gamma rays X rays ultraviolet rays visible light infrared waves microwaves and radio waves. What is the difference between a continuous spectrum and a bright-line emission spectrum. And clean concrete roads are light in tone.
Note that spectral lines can also occur in other regions of the electromagnetic spectrum. Emission lines refer to the fact that glowing hot gas emits lines of light whereas absorption lines refer to the tendency of cool atmospheric gas to absorb the same lines of light. If all the seven colors are present with no gaps between them it makes a continuous spectrum.
In general darker shades of each color indicate moister soil. The emission spectrum of hydrogen is divided into a number of spectral lines with wavelengths given by the Rydberg formula. Red is also a color of Christmas bringing joy warmth and safety.
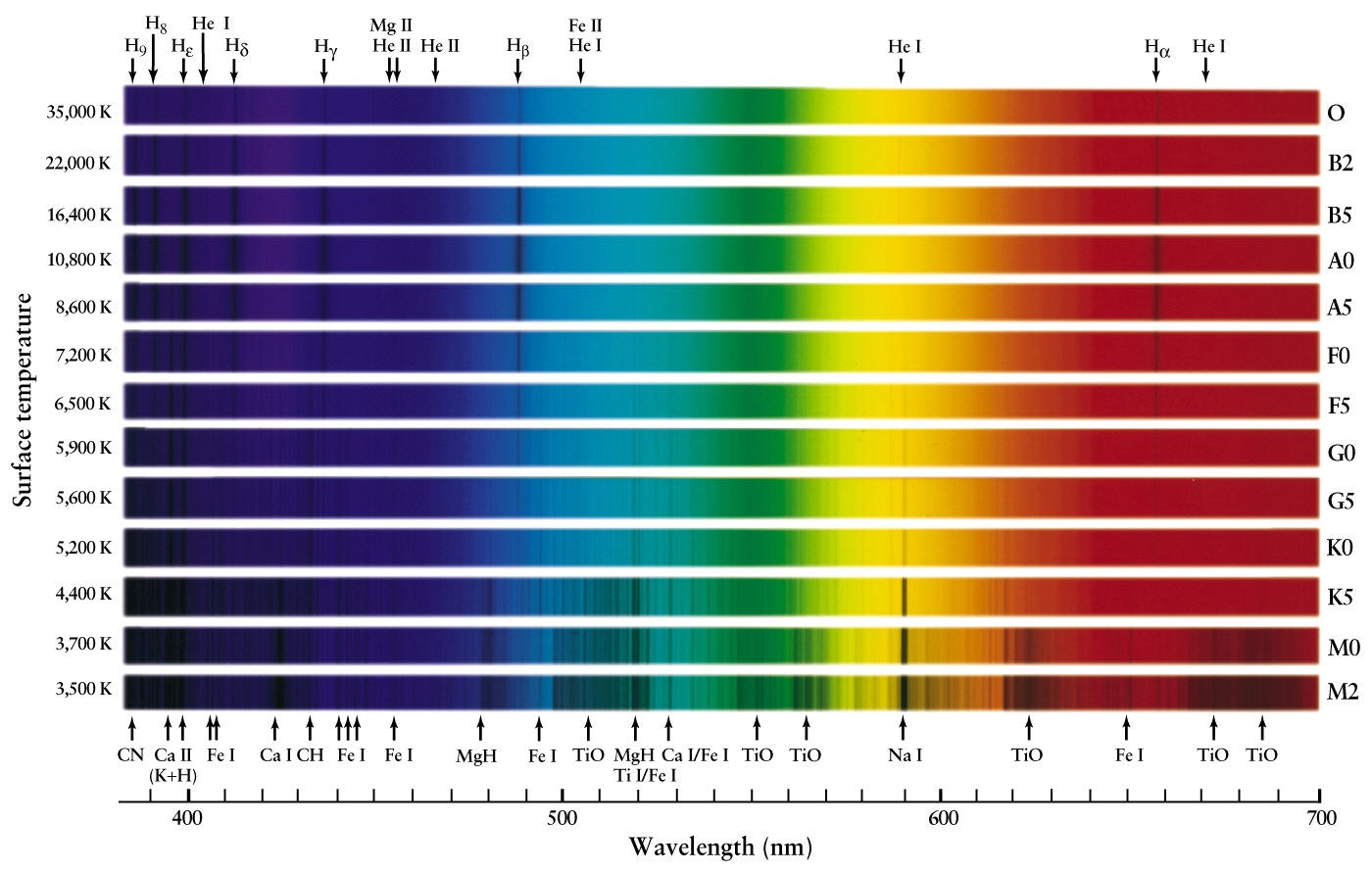
Colors of light that correspond to narrow wavelength bands monochromatic light are the pure spectral colors learned using the ROYGBIV acronym. The Emission Spectra of Elements Compared with Hydrogen. The visible spectrum or color spectrum is a subset of the electromagnetic spectrum.
These observed spectral lines are due to the electron making transitions between the energy levels in an atom. The different colours in a line spectrum represent different frequencies of light. A continuous spectrum shows all the colors of the rainbow.
The visible light frequencies lie between the frequencies of the ultraviolet rays and infrared waves. These images show a. When light passes through gas in the atmosphere some of the light at particular wavelengths is.
When this happens an absorption-line spectrum will be produced. The different colors in a line spectrum represents when an electron falls from one energy level in an atom to a lower energy level it emits a photon of a particular wavelength and energy. The lighter the colour will represent light being BOUNCED off a surface and a darker colour will represent light.
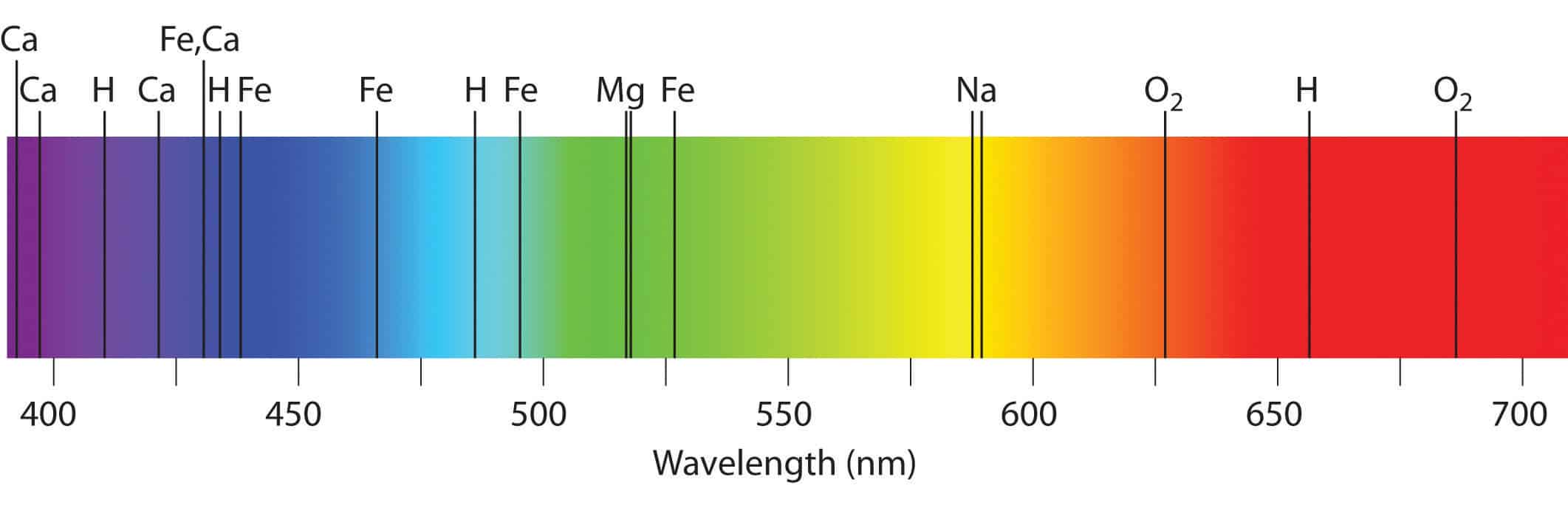
These so called line spectra are characteristic of the atomic composition of the gas. Red has a wavelength of 7800-6220 angstroms green has a wavelength of 5770-4920 angstroms and violet has a wavelength. For example light pastel peach tones are seen as sweet conversational and affable whereas more intense vibrant oranges are seen as representative of vitality energy and encouragement.
Each element produces its own unique line spectrum. White light is believed to contain all the seven rainbow colors which are absorbed at different wavelengths when refracted in a prism. Solve any question of Structure of Atom with-.
Bohr had an answer. Red orange yellow green blue indigo and violet. So why do energized hydrogen gas molecules produce a line spectrum but not a continuous spectrum.
With sodium however we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum at about 589 nm. Many of us consider red as a color of love but in fact it is more related to the excitement of falling in love and awaking passion for the other person. The spectra for each element is unique because each element contains a different number of electrons and thus different energy levels.
All of the visible light wavelengths. What do the different colors in a line spectrum represent. On the other hand it stands for aggression danger violence and war.
Man-made features appear in tones that relate to the materials with which they are made. The different colors in a line spectrum represent the number of transitions in each atom and its energy levels. That is why that when the energy level rises from the excitement to heat the element begins to produce the light according to its reaction to the energy level and line of spectrum.
An angstrom is 10 -8 cm or 000000001 cm. Gravel or dirt roads are lighter colors depending on their composition. Because each element has a different set of emission colors from the emission spectrum.
The true wavelengths are actually measured in terms of angstroms. Photons of different wavelengths and therefore different colors are released from each element. The light emitted by hydrogen atoms is red because of its four characteristic lines the most intense line in its spectrum is in the red portion of the visible spectrum at 656 nm.
It has been calculated that the observed colors in a hydrogen atom correspond to the relaxation of the electron from a higher energy level to the second energy level. What do the individual lines in a bright line emission spectrum represent. Each element has its own unique line spectrum and is thus referred to as the fingerprint for a particular element.
Transitions in each atom and the energy levels in it photons of different wavelengths and thus different colors are released from each gas. It can symbolize desire power speed and strength.

4 2 Understanding Atomic Spectra Chemistry Libretexts

No comments for "Explain What the Different Colors in a Line Spectrum Represent"
Post a Comment